In a major scientific breakthrough, researchers have developed an innovative photocatalyst capable of efficiently degrading sulfamethoxazole (SMX), a widely used antibiotic. This discovery could provide a sustainable solution to the growing issue of antibiotic contamination in the environment, which poses significant threats to both ecosystems and human health.
Why is Antibiotic Contamination a Problem?
The presence of antibiotics like SMX in water and soil can lead to a variety of environmental and health concerns, including:
- Antibiotic Resistance: Continuous exposure to antibiotics in the environment accelerates the development of antibiotic-resistant bacteria. This makes it harder to treat infections, as these bacteria become immune to standard treatments, posing a serious global health risk.
- Ecological Disruption: Antibiotics can interfere with the balance of ecosystems, negatively impacting plants, animals, and essential microorganisms. This disruption can lead to biodiversity loss and weakened ecosystems.
- Human Health Risks: Prolonged exposure to antibiotic residues in contaminated water sources has the potential to affect human health. Studies have shown links between low-level antibiotic exposure and various health issues, including hormonal imbalances and the spread of resistant pathogens.
The Development of the Catalyst
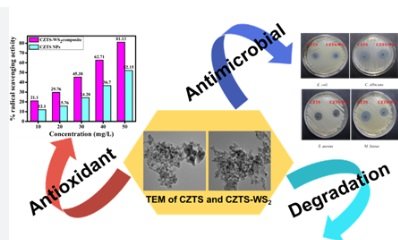
The team of scientists, under the leadership of Prof. Devasish Chowdhury at the Institute of Advanced Study in Science and Technology (IASST), designed the catalyst by utilizing a combination of earth-abundant materials. The composite catalyst is made from copper zinc tin sulfide (CZTS) and tungsten disulfide (WS2) nanoparticles. The key steps in the catalyst’s creation involved:
- Using environmentally friendly and non-toxic chemicals like zinc chloride, copper chloride, and tin chloride, combined with tungsten disulfide.
- Employing a hydrothermal reaction to process these materials, which involved heating them in water, resulting in the formation of the catalyst.
One of the standout features of this new catalyst is that it is derived from readily available, cost-effective materials, making it not only safe for the environment but also economically viable.
How Does It Work?
The newly developed CZTS-WS2 composite works by breaking down SMX into less harmful by-products, thus reducing its toxic impact on the environment. Here’s a closer look at its key features:
- Degradation Mechanism: The catalyst initiates a chemical reaction that breaks down SMX, transforming it into safer substances. This process is crucial in mitigating the harmful effects of antibiotic residues in water and soil.
- Reusability: Unlike some catalysts that lose their efficiency after a few uses, this new composite can be reused multiple times without significant loss of performance. This makes it highly cost-efficient for large-scale applications.
Testing and Performance
The efficacy of the catalyst was rigorously tested using Liquid Chromatography-Mass Spectrometry (LC-MS). This advanced technique allowed the researchers to monitor the breakdown process and identify the substances formed as intermediates. The results revealed that the catalyst successfully degraded SMX into less harmful compounds, significantly reducing its environmental impact.
In terms of performance, the catalyst demonstrated impressive capabilities:
- Radical Scavenging: It achieved over 80% efficiency in scavenging free radicals—unstable molecules that can cause environmental and biological damage.
- Antibacterial Properties: In addition to breaking down antibiotics, the catalyst also exhibited antibacterial activity, making it effective in reducing harmful bacteria in the environment.
Summing Up
The development of the CZTS-WS2 composite represents a promising leap forward in the fight against antibiotic pollution. As antibiotic residues in water and soil continue to pose a growing threat to ecosystems and human health, this breakthrough could pave the way for more sustainable and effective environmental management practices. The reusability, cost-effectiveness, and efficiency of the catalyst make it an attractive solution for mitigating the risks associated with antibiotic contamination, offering hope for a cleaner, healthier future.

